Temperature and Absolute Zero
 We all know that some things feel hot, and others cold, but is there more to
temperature than that?
We all know that some things feel hot, and others cold, but is there more to
temperature than that?
 When an object feels hot, the atoms inside it are moving fast in random
directions, and when it feels cold, they are moving slowly. Our body
interprets that random atomic motion into what we feel as hot and cold, and a
thermometer interprets that atomic motion as a certain number of degrees.
When an object feels hot, the atoms inside it are moving fast in random
directions, and when it feels cold, they are moving slowly. Our body
interprets that random atomic motion into what we feel as hot and cold, and a
thermometer interprets that atomic motion as a certain number of degrees.
 So when I'm heating something, I'm just making its atoms move faster?
So when I'm heating something, I'm just making its atoms move faster?
 Exactly. If the object is a solid the atoms are vibrating back and forth, and
if it is a gas like the air, the atoms are flying around much like little
balls.
Exactly. If the object is a solid the atoms are vibrating back and forth, and
if it is a gas like the air, the atoms are flying around much like little
balls.
 They are bouncing around so that sometimes an atom is going fast, and other
times it is slow. It seems like its temperature must be changing all the
time.
They are bouncing around so that sometimes an atom is going fast, and other
times it is slow. It seems like its temperature must be changing all the
time.
 In a group of atoms there is always a whole range of speeds, but while the
speed of one atom changes, the average of all of them does not. See how each
time an atom slows down, there has to be another that speeds up? So
temperature is really describing the range of speeds of the bunch of atoms
together. Physicists often like to use a different scale for temperature that
is directly related to the speed atoms are going in a gas. This is called the
Absolute scale, and one degree on it is the same as one degree centigrade,
which is 9/5 of a degree Fahrenheit. The difference between the Centigrade and
Absolute scales is the zero label.
In a group of atoms there is always a whole range of speeds, but while the
speed of one atom changes, the average of all of them does not. See how each
time an atom slows down, there has to be another that speeds up? So
temperature is really describing the range of speeds of the bunch of atoms
together. Physicists often like to use a different scale for temperature that
is directly related to the speed atoms are going in a gas. This is called the
Absolute scale, and one degree on it is the same as one degree centigrade,
which is 9/5 of a degree Fahrenheit. The difference between the Centigrade and
Absolute scales is the zero label.
 What is so "Absolute" about it?
What is so "Absolute" about it?
|
|
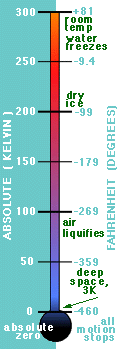
 So that is as cold as the atoms can be. We call that Absolute Zero.
So that is as cold as the atoms can be. We call that Absolute Zero.
 I get it! When the atoms are all stopped the gas is ABSOLUTELY as cold as can
be!
I get it! When the atoms are all stopped the gas is ABSOLUTELY as cold as can
be!
 Yes, and that is really cold. The thermometer shows a comparison of the
Absolute (also known as the Kelvin) and Fahrenheit scales of temperature.
Absolute Zero is -459 degrees Fahrenheit.
Yes, and that is really cold. The thermometer shows a comparison of the
Absolute (also known as the Kelvin) and Fahrenheit scales of temperature.
Absolute Zero is -459 degrees Fahrenheit.
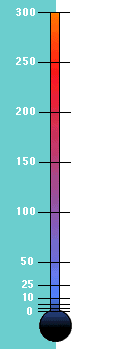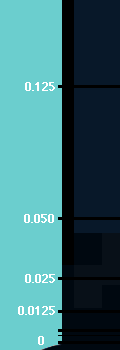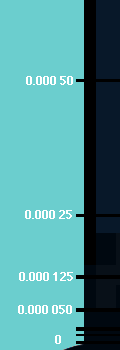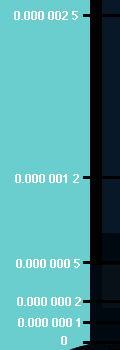
|
 Is anything actually at Absolute Zero?
Is anything actually at Absolute Zero?
|
 That is a really interesting question. It turns out that the heat left over
from the Big Bang that created the universe is everywhere, and it keeps the
temperature in space from going any lower than 3 degrees Kelvin. Measuring this temperature is the strongest evidence we
have that the Big Bang actually happened. However, people can do a
lot better than nature when it comes to getting things cold. For almost a
century we have been able to build refrigerators that get to lower than 3
degrees above Absolute Zero, and for quite a while we have been able to even
get lower than 1/1000 of a degree above Absolute Zero. However, a big
step was when Cornell and Wieman cooled a small sample of atoms down to only a
few billionths (0.000,000,001) of a degree above Absolute Zero! That
was what they needed to do to see Bose-Einstein condensation.
That is a really interesting question. It turns out that the heat left over
from the Big Bang that created the universe is everywhere, and it keeps the
temperature in space from going any lower than 3 degrees Kelvin. Measuring this temperature is the strongest evidence we
have that the Big Bang actually happened. However, people can do a
lot better than nature when it comes to getting things cold. For almost a
century we have been able to build refrigerators that get to lower than 3
degrees above Absolute Zero, and for quite a while we have been able to even
get lower than 1/1000 of a degree above Absolute Zero. However, a big
step was when Cornell and Wieman cooled a small sample of atoms down to only a
few billionths (0.000,000,001) of a degree above Absolute Zero! That
was what they needed to do to see Bose-Einstein condensation.
|

|



 Well, look what happens if you set the temperature of the atoms in the box as
low as it can go.
Well, look what happens if you set the temperature of the atoms in the box as
low as it can go.
 The atoms are stopped.
The atoms are stopped.
 The coldest place in nature is the depths of outer space. There it is 3
degrees above Absolute Zero.
The coldest place in nature is the depths of outer space. There it is 3
degrees above Absolute Zero.
 Why doesn't it get down all the way to Absolute Zero?
Why doesn't it get down all the way to Absolute Zero?



