What does a Bose-Einstein condensate look like?
 It looks like a dense little lump in the bottom of the magnetic trap/bowl;
kind of like a drop of water condensing out of damp air onto a cold bowl.
When it first forms, though, the condensate is still surrounded by the normal
gas atoms, so it looks a bit like a pit inside a cherry.
It looks like a dense little lump in the bottom of the magnetic trap/bowl;
kind of like a drop of water condensing out of damp air onto a cold bowl.
When it first forms, though, the condensate is still surrounded by the normal
gas atoms, so it looks a bit like a pit inside a cherry.
|

|
 Could I just look into the experiment and see it?
Could I just look into the experiment and see it?
 Yes, but it is quite a small lump, so you need to use a microscope. Also, you
would have to illuminate it with the special deep red color that the rubidium
atom reflects. (Click here to
learn more about atoms and the colors to which they respond.) The way Wieman
and Cornell first looked at it was to turn off the trap, and then, after a
little while, take a snapshot picture of the cloud. When they got the cloud
cold enough, they could see a very dense blob form in the center. You can see
this in the pictures of their actual data as they cool the atoms from 400
billionths of a degree above absolute zero down to 50 billionths.
Yes, but it is quite a small lump, so you need to use a microscope. Also, you
would have to illuminate it with the special deep red color that the rubidium
atom reflects. (Click here to
learn more about atoms and the colors to which they respond.) The way Wieman
and Cornell first looked at it was to turn off the trap, and then, after a
little while, take a snapshot picture of the cloud. When they got the cloud
cold enough, they could see a very dense blob form in the center. You can see
this in the pictures of their actual data as they cool the atoms from 400
billionths of a degree above absolute zero down to 50 billionths.
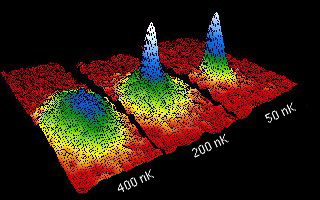
Click to view larger image. Grey scale has been converted to color, with white
indicating densest (and hence darkest) part of the cloud. To see a false-color
movie showing an actual cloud of atoms cooling down and condensing, click on
one of the following:
|
BEC QuickTime movie, 850 Kbytes | OR |
BEC movie animated gif, 2.8 Mbytes |
 So the atoms on the edges of the picture are spreading out, but the
Bose-Einstein condensate peak in the middle doesn't?
So the atoms on the edges of the picture are spreading out, but the
Bose-Einstein condensate peak in the middle doesn't?
 You are right about the atoms on the side, and almost right about the
condensate. It actually does spread out, but the way it spreads out shows some
of the reasons it is quite a special little lump.
You are right about the atoms on the side, and almost right about the
condensate. It actually does spread out, but the way it spreads out shows some
of the reasons it is quite a special little lump.
 I was beginning to wonder what the big deal is over just a little lump.
I was beginning to wonder what the big deal is over just a little lump.
 This lump spreads out as slowly as any atoms possibly can that are not stuck
together, as they are in any solid material. There is a basic law of physics
requiring them to spread called "the Heisenberg uncertainty principle" that
says you cannot simultaneously know the exact location and the exact speed of
anything, including atoms. Since we can see about where they are located, then
we can't know exactly how fast they are moving. If they were stationary, we
would know they were moving with zero speed. So that's why they spread out.
But to really understand the uncertainty principle is another story.
This lump spreads out as slowly as any atoms possibly can that are not stuck
together, as they are in any solid material. There is a basic law of physics
requiring them to spread called "the Heisenberg uncertainty principle" that
says you cannot simultaneously know the exact location and the exact speed of
anything, including atoms. Since we can see about where they are located, then
we can't know exactly how fast they are moving. If they were stationary, we
would know they were moving with zero speed. So that's why they spread out.
But to really understand the uncertainty principle is another story.






