Quantum Numbers and Angular Momentum
 So a larger value of l means more angular momentum?
So a larger value of l means more angular momentum?
 That's right. If you really want to know the exact mathematical
relationship between l and angular momentum, click on the advanced
button.
That's right. If you really want to know the exact mathematical
relationship between l and angular momentum, click on the advanced
button.
|

|
 We've seen that states with higher values of l have more energy--p
states have a higher energy than s states, and so on. I'm not quite sure why
more angular momentum means more energy.
We've seen that states with higher values of l have more energy--p
states have a higher energy than s states, and so on. I'm not quite sure why
more angular momentum means more energy.
 Well, the larger its angular momentum, the farther from the nucleus an
electron tends to be.
Well, the larger its angular momentum, the farther from the nucleus an
electron tends to be.
 That makes sense--the same thing is true for planets orbiting the sun, right?
I know electrons don't orbit exactly, but...
That makes sense--the same thing is true for planets orbiting the sun, right?
I know electrons don't orbit exactly, but...
 No, your intuition is good. It's difficult to see how the idea of angular
momentum even applies to something that's just existing amorphously in a
cloud, and yet it has to; the theory simply won't work without it. To have
any picture of what's happening, we have to fall back on that same old
wrong-but-useful orbiting idea.
No, your intuition is good. It's difficult to see how the idea of angular
momentum even applies to something that's just existing amorphously in a
cloud, and yet it has to; the theory simply won't work without it. To have
any picture of what's happening, we have to fall back on that same old
wrong-but-useful orbiting idea.
Now, suppose you're an electron with lots of angular
momentum, floating out there beyond your less well-endowed friends. Any
electrons between you and the nucleus are "screening" you from the full
effect of the central positive charge. Think about boron, for example; the
two innermost electrons feel all five protons pulling them in, but the
highest electron "sees" four electrons between it and the five protons, so
the total charge it feels is hardly more than that of one proton.

 And the less strongly it's pulled in, the less energy it needs to escape --so
that's why a larger l means a higher energy.
And the less strongly it's pulled in, the less energy it needs to escape --so
that's why a larger l means a higher energy.
What about that third quantum number you mentioned? Is
m also related to angular momentum?

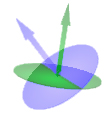 Yes; m refers, loosely, to the direction of the angular
momentum vector. (Want an exact formula?) As you might expect, m
doesn't affect the electron's energy, but it does affect the probability
cloud.
Yes; m refers, loosely, to the direction of the angular
momentum vector. (Want an exact formula?) As you might expect, m
doesn't affect the electron's energy, but it does affect the probability
cloud.




