Energy Levels
 So that's it? Atoms really look like little solar systems with electrons making quantum
jumps between special orbits?
So that's it? Atoms really look like little solar systems with electrons making quantum
jumps between special orbits?
 Well, not quite. The idea of an electron actually flying around in little circles turned out
to have lots of problems, and physicists were eventually forced to discard that model.
Well, not quite. The idea of an electron actually flying around in little circles turned out
to have lots of problems, and physicists were eventually forced to discard that model.
 But we just finished talking about how well that worked! Why do we have to throw the whole
thing away?
But we just finished talking about how well that worked! Why do we have to throw the whole
thing away?
 We're not going to start from scratch. The concept of "special orbits" was extremely useful,
it's just the orbits themselves that we're not going to use anymore. Instead, we're going to
think about electrons being in special energy levels. We just use this rule:
We're not going to start from scratch. The concept of "special orbits" was extremely useful,
it's just the orbits themselves that we're not going to use anymore. Instead, we're going to
think about electrons being in special energy levels. We just use this rule:
 Oh, that's easy enough. But why bother? Why not just call them orbits?
Oh, that's easy enough. But why bother? Why not just call them orbits?
 Well, first of all, some orbits have the same energy as other orbits, so sometimes changing
orbits wouldn't emit radiation. Also, it turns out that electrons don't really move in
little circular orbits. We can take a little detour to see how the
Schrödinger Atom more accurately depicts what is happening inside atoms.
Well, first of all, some orbits have the same energy as other orbits, so sometimes changing
orbits wouldn't emit radiation. Also, it turns out that electrons don't really move in
little circular orbits. We can take a little detour to see how the
Schrödinger Atom more accurately depicts what is happening inside atoms.
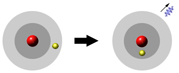
 Actually, thinking about energy levels makes more sense, anyway, because if the energy goes
down the extra energy has to go somewhere, so it comes out as electromagnetic
radiation.
Actually, thinking about energy levels makes more sense, anyway, because if the energy goes
down the extra energy has to go somewhere, so it comes out as electromagnetic
radiation.
|
|
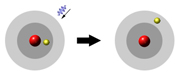
 Yeah, and in order for the energy to go up it has to come from somewhere, so it
takes some incoming radiation!
Yeah, and in order for the energy to go up it has to come from somewhere, so it
takes some incoming radiation!
|
|
 This next applet shows the Bohr model along with a diagram showing the energy level. This
"energy level" picture of an atom is so useful that most physicists prefer it over the orbital picture.
This next applet shows the Bohr model along with a diagram showing the energy level. This
"energy level" picture of an atom is so useful that most physicists prefer it over the orbital picture.
 Hold on. Earlier we were saying that when an electron changes its speed or direction, it
gives off electromagnetic radiation. Now we're saying that when an electron changes its orbit
(or "energy level") it gives off electromagnetic radiation. Which is it?
Hold on. Earlier we were saying that when an electron changes its speed or direction, it
gives off electromagnetic radiation. Now we're saying that when an electron changes its orbit
(or "energy level") it gives off electromagnetic radiation. Which is it?
 You're changing your story on us! Are you making this up as you go along or what?
You're changing your story on us! Are you making this up as you go along or what?
 Change in velocity was a classical idea, but the quantum physicists realized the important
part is that the energy of the electron changes, and electromagnetic radiation makes up
the difference. If the energy goes down, the extra energy appears as a photon. And for the
electron to get more energy, it needs to absorb a photon. Now let's look at how this
theory neatly explains spectral lines...
Change in velocity was a classical idea, but the quantum physicists realized the important
part is that the energy of the electron changes, and electromagnetic radiation makes up
the difference. If the energy goes down, the extra energy appears as a photon. And for the
electron to get more energy, it needs to absorb a photon. Now let's look at how this
theory neatly explains spectral lines...






