Circular Waves and the Bohr Model
 De Broglie proposed that every particle has a wavelength equal to Planck's
constant divided by its momentum. Thus, an electron with mass m and
speed v would have a wavelength of
De Broglie proposed that every particle has a wavelength equal to Planck's
constant divided by its momentum. Thus, an electron with mass m and
speed v would have a wavelength of
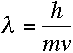
|
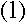
|
Now, if the wave has to fit into a circular "orbit" of radius r, the
circumference of that circle must be exactly as long as a whole number of
wavelengths:
|
|
where n is a positive integer. Plugging in the wavelength from
equation (1), we get
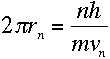
|
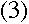
|
or
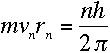
|
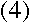
|
But we know that for a circular orbit,
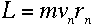
|
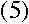
|
Thus, if we assume the electron is a wave with its wavelength given by de
Broglie's formula, then we automatically get Bohr's constraint on its angular
momentum:
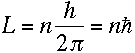
|
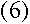
|
The integer n, as we've noted earlier, corresponds to the energy level;
thus, for example, an electron in the third energy level would have three
wavelengths around its orbit, and so on.





