Making X-rays
 Where do x-rays come from?
Where do x-rays come from?
 An x-ray machine, like that used in a doctor's or a dentist's office, is
really very simple. Inside the machine is an x-ray tube. An electron gun
inside the tube shoots high energy electrons at a target made of heavy
atoms, such as tungsten. X-rays come out because of atomic processes induced by the
energetic electrons shot at the target.
An x-ray machine, like that used in a doctor's or a dentist's office, is
really very simple. Inside the machine is an x-ray tube. An electron gun
inside the tube shoots high energy electrons at a target made of heavy
atoms, such as tungsten. X-rays come out because of atomic processes induced by the
energetic electrons shot at the target.
|
|
 X-rays are just like any other kind of electromagnetic radiation. They can be
produced in parcels of energy called photons, just like light. There are two
different atomic processes that can produce x-ray photons. One is called
Bremsstrahlung, which is a fancy German name meaning "braking
radiation." The other is called K-shell emission. They can both occur
in heavy atoms like tungsten.
X-rays are just like any other kind of electromagnetic radiation. They can be
produced in parcels of energy called photons, just like light. There are two
different atomic processes that can produce x-ray photons. One is called
Bremsstrahlung, which is a fancy German name meaning "braking
radiation." The other is called K-shell emission. They can both occur
in heavy atoms like tungsten.
 So do both ways of making x-rays involve a change in the state of electrons?
So do both ways of making x-rays involve a change in the state of electrons?
 That's right. But Bremsstrahlung is easier to understand using the classical
idea that radiation is emitted when the velocity of the electron shot at the
tungsten changes. This electron slows down after swinging around the nucleus
of a tungsten atom and loses energy by radiating x-rays. In the quantum
picture, a lot of photons of different wavelengths are produced, but none of
the photons has more energy than the electron had to begin with. After
emitting the spectrum of x-ray radiation the original electron is slowed down
or stopped.
That's right. But Bremsstrahlung is easier to understand using the classical
idea that radiation is emitted when the velocity of the electron shot at the
tungsten changes. This electron slows down after swinging around the nucleus
of a tungsten atom and loses energy by radiating x-rays. In the quantum
picture, a lot of photons of different wavelengths are produced, but none of
the photons has more energy than the electron had to begin with. After
emitting the spectrum of x-ray radiation the original electron is slowed down
or stopped.
 What is the "K-shell" in the other way of making x-rays?
What is the "K-shell" in the other way of making x-rays?
 Do you remember that atoms have their electrons arranged in closed "shells" of
different energies? Well, the K-shell is the lowest energy state of an atom.
Do you remember that atoms have their electrons arranged in closed "shells" of
different energies? Well, the K-shell is the lowest energy state of an atom.
 What can the incoming electron from the electron gun do to a K-shell
electron in a tungsten target atom?
What can the incoming electron from the electron gun do to a K-shell
electron in a tungsten target atom?
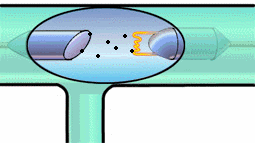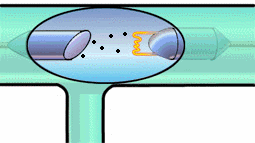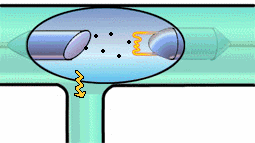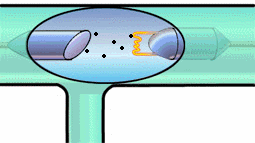
 It can give it enough energy to knock it out of its energy state. Then, a
tungsten electron of higher energy (from an outer shell) can fall into the
K-shell. The energy lost by the falling electron shows up in an emitted x-ray
photon. Meanwhile, higher energy electrons fall into the vacated energy state
in the outer shell, and so on. K-shell emission produces higher-intensity
x-rays than Bremsstrahlung, and the x-ray photon comes out at a single
wavelength. Have a look at both mechanisms in the experiment below.
It can give it enough energy to knock it out of its energy state. Then, a
tungsten electron of higher energy (from an outer shell) can fall into the
K-shell. The energy lost by the falling electron shows up in an emitted x-ray
photon. Meanwhile, higher energy electrons fall into the vacated energy state
in the outer shell, and so on. K-shell emission produces higher-intensity
x-rays than Bremsstrahlung, and the x-ray photon comes out at a single
wavelength. Have a look at both mechanisms in the experiment below.
|
|
|






