Atomic Weight
 How did Mendeleev know the weights of different atoms?
How did Mendeleev know the weights of different atoms?

 Good question. Nineteenth century chemists couldn't take
individual atoms and plunk them on a scale. They could only study
chemical reactions involving huge numbers of atoms all at once.
Good question. Nineteenth century chemists couldn't take
individual atoms and plunk them on a scale. They could only study
chemical reactions involving huge numbers of atoms all at once.
 So how would they ever find the weight of one atom by itself?
So how would they ever find the weight of one atom by itself?
 Well, by the early 1800's, scientists like John Dalton had discovered that the weights of
elements involved in chemical reactions always have to be in certain
proportions.
Well, by the early 1800's, scientists like John Dalton had discovered that the weights of
elements involved in chemical reactions always have to be in certain
proportions.
|

|
| For example: hydrogen and oxygen combine to form water, H2O. If you don't want any leftover hydrogen or oxygen, then the weight of oxygen you start with has to be eight times the weight of the hydrogen. |
 So that lets you calculate the weight of one atom? I don't quite
see how.
So that lets you calculate the weight of one atom? I don't quite
see how.
 Think about what's going on in the reaction: two hydrogen atoms and one oxygen
atom join together to make one water molecule. So if you want to end up with
nothing but water molecules, you have to start with atoms in the right
proportion: two hydrogens for every oxygen.
Think about what's going on in the reaction: two hydrogen atoms and one oxygen
atom join together to make one water molecule. So if you want to end up with
nothing but water molecules, you have to start with atoms in the right
proportion: two hydrogens for every oxygen.
 I see! Then one oxygen atom must weigh eight times as much as two
hydrogens.
I see! Then one oxygen atom must weigh eight times as much as two
hydrogens.
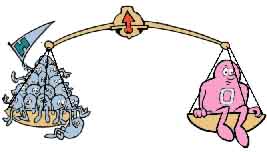
 Exactly. Mendeleev and his contemporaries couldn't say how much a given
atom weighed, in pounds or grams, but, by
studying reactions in this way, they could tell you how heavy it was in
relation to other atoms. One oxygen atom weighs as much as sixteen
hydrogens; carbon, twelve hydrogens; helium, four, and so on. Thus
Mendeleev was able to arrange the elements in order from lightest to
heaviest.
Exactly. Mendeleev and his contemporaries couldn't say how much a given
atom weighed, in pounds or grams, but, by
studying reactions in this way, they could tell you how heavy it was in
relation to other atoms. One oxygen atom weighs as much as sixteen
hydrogens; carbon, twelve hydrogens; helium, four, and so on. Thus
Mendeleev was able to arrange the elements in order from lightest to
heaviest.




