The Origin of the Periodic Table
 I know what the periodic table looks like, but where did it come from?
Whose idea was it to arrange the elements this way?
I know what the periodic table looks like, but where did it come from?
Whose idea was it to arrange the elements this way?
 In 1869, a Russian chemist named Dmitri
Mendeleev came up with a way of organizing the elements that were
known at the time.
In 1869, a Russian chemist named Dmitri
Mendeleev came up with a way of organizing the elements that were
known at the time.
|

|
 1869...that's way before the
Schrödinger model, or even the Rutherford model.
1869...that's way before the
Schrödinger model, or even the Rutherford model.
 That's right. Mendeleev had no idea what atoms were made of or why they
behaved as they did. Nevertheless, he was able to put together the periodic
table almost as we know it today--except that some elements were missing,
because they were unknown in 1869.
That's right. Mendeleev had no idea what atoms were made of or why they
behaved as they did. Nevertheless, he was able to put together the periodic
table almost as we know it today--except that some elements were missing,
because they were unknown in 1869.
 Based on the gaps in his table, Mendeleev even succeeded in predicting the
existence and properties of several new elements.
Based on the gaps in his table, Mendeleev even succeeded in predicting the
existence and properties of several new elements.
 That's pretty impressive. Can you tell me more about how Mendeleev
organized the table? What kinds of properties did he use?
That's pretty impressive. Can you tell me more about how Mendeleev
organized the table? What kinds of properties did he use?
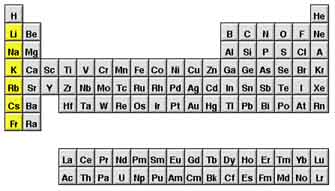
 His basic rule was this: the elements in any column, or group, of the
table are similar to their column-mates. For example, look at the first
column on the left, underneath hydrogen (H). The elements in this group are
called the alkali metals; they're all
soft metals that react violently with water to make hydrogen gas.
His basic rule was this: the elements in any column, or group, of the
table are similar to their column-mates. For example, look at the first
column on the left, underneath hydrogen (H). The elements in this group are
called the alkali metals; they're all
soft metals that react violently with water to make hydrogen gas.
 Click here if you'd like to read more about Mendeleev's
methods and the chemistry of his time.
Click here if you'd like to read more about Mendeleev's
methods and the chemistry of his time.





