Periodic Properties
 Hmm...so the elements in the table are arranged in order from lightest to heaviest, and elements in the
same column have matching properties.
Hmm...so the elements in the table are arranged in order from lightest to heaviest, and elements in the
same column have matching properties.
|
 That's right. That's right.
|
 That means that elements whose atomic weights are really close together
can be very different, and some elements with far-apart atomic weights are
very similar. As you move from lighter atoms to heavier ones, you keep
periodically running across the same properties...
That means that elements whose atomic weights are really close together
can be very different, and some elements with far-apart atomic weights are
very similar. As you move from lighter atoms to heavier ones, you keep
periodically running across the same properties...
 Hence the name periodic table.
Hence the name periodic table.
|
 Oh, right.
Oh, right.
|
 I don't mean to pick on you; what you said was actually a very important
insight. The periodic table is full of repeating patterns. Take atomic size, for instance: atoms get bigger as you move
down a column, and smaller as you move to the right across a row, or
period.
I don't mean to pick on you; what you said was actually a very important
insight. The periodic table is full of repeating patterns. Take atomic size, for instance: atoms get bigger as you move
down a column, and smaller as you move to the right across a row, or
period.
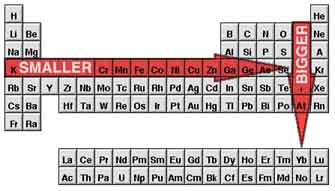
 That's so weird! I'd think the atoms would just get bigger as
they got heavier; why do they get smaller as you move to
the right?
That's so weird! I'd think the atoms would just get bigger as
they got heavier; why do they get smaller as you move to
the right?
 The answer lies in the underlying structure of those atoms...
The answer lies in the underlying structure of those atoms...






