Units
 This brings up the issue of units. In your math class, numbers by themselves are fine
to work with, but in science a number without units is pretty useless. Try this
problem: a train of length 2, weighing 200, travels from Denver to Santa Fe at 15. How
long did the trip take?
This brings up the issue of units. In your math class, numbers by themselves are fine
to work with, but in science a number without units is pretty useless. Try this
problem: a train of length 2, weighing 200, travels from Denver to Santa Fe at 15. How
long did the trip take?
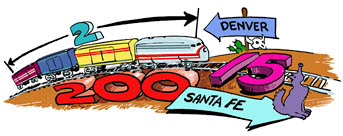
 What? 2 kilometers, 200 tons, 15 miles per hour?
What? 2 kilometers, 200 tons, 15 miles per hour?
 That's right, we have no clue unless we put units on those numbers.
That's right, we have no clue unless we put units on those numbers.
 Okay, but what in the world is
Okay, but what in the world is 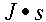 ?
?
 The way you build a unit system is to have a fundamental set of units - things like
length, mass, time, and temperature, to name a few. In the standard system of units
used by scientists, we measure those quantities in meters [m], kilograms [kg], seconds
[s], and Kelvin [K] respectively.
The way you build a unit system is to have a fundamental set of units - things like
length, mass, time, and temperature, to name a few. In the standard system of units
used by scientists, we measure those quantities in meters [m], kilograms [kg], seconds
[s], and Kelvin [K] respectively.
|

|
 I see that the s stands for seconds, but where did that J come from, Dr. Mahan?
I see that the s stands for seconds, but where did that J come from, Dr. Mahan?
 That J stands for Joule, in honor of James Joule who
examined the relationship between heat and energy in the 19th Century. The Joule is the
SI unit for measuring energy. A joule is related to the fundamental units in that a
joule is 1 kg m2/s2. By no means is that obvious, but it is
similar to measuring area in m2 (distance times distance) or speed in m/s
(distance divided by time).
That J stands for Joule, in honor of James Joule who
examined the relationship between heat and energy in the 19th Century. The Joule is the
SI unit for measuring energy. A joule is related to the fundamental units in that a
joule is 1 kg m2/s2. By no means is that obvious, but it is
similar to measuring area in m2 (distance times distance) or speed in m/s
(distance divided by time).

 So
So 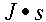 is a Joule second. What would that be?
That's not quite energy, not quite time.
is a Joule second. What would that be?
That's not quite energy, not quite time.
 The fancy name for what a Joule represents is angular momentum, but all that you need
to know now is that it involves things that spin, like bicycle wheels and tops.
The fancy name for what a Joule represents is angular momentum, but all that you need
to know now is that it involves things that spin, like bicycle wheels and tops.
 Weird. And that has something to do with photons?
Weird. And that has something to do with photons?
 Yep, there's a lot we haven't told you about light.
Yep, there's a lot we haven't told you about light.





