Planck's Constant and the Energy of a Photon
 In 1900, Max Planck was working on the problem of how the radiation an object emits is
related to its temperature. He came up with a formula that agreed very closely with
experimental data, but the formula only made sense if he assumed that the energy of a
vibrating molecule was quantized--that is, it could only take on certain values.
The energy would have to be proportional to the frequency of vibration, and it seemed
to come in little "chunks" of the frequency multiplied by a certain constant. This
constant came to be known as Planck's constant, or h, and it has the
value
In 1900, Max Planck was working on the problem of how the radiation an object emits is
related to its temperature. He came up with a formula that agreed very closely with
experimental data, but the formula only made sense if he assumed that the energy of a
vibrating molecule was quantized--that is, it could only take on certain values.
The energy would have to be proportional to the frequency of vibration, and it seemed
to come in little "chunks" of the frequency multiplied by a certain constant. This
constant came to be known as Planck's constant, or h, and it has the
value
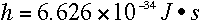
|

| This doesn't make any sense to me. I think I'll go ask Dr. Mahan what J means. |
 That's a pretty small constant.
That's a pretty small constant.
 Yes, but it was an extremely radical idea to suggest that energy could only come in
discrete lumps, even if the lumps were very small. Planck actually didn't realize how
revolutionary his work was at the time; he thought he was just fudging the math to come
up with the "right answer," and was convinced that someone else would come up with a
better explanation for his formula.
Yes, but it was an extremely radical idea to suggest that energy could only come in
discrete lumps, even if the lumps were very small. Planck actually didn't realize how
revolutionary his work was at the time; he thought he was just fudging the math to come
up with the "right answer," and was convinced that someone else would come up with a
better explanation for his formula.
 I guess Einstein must have taken him seriously, though.
I guess Einstein must have taken him seriously, though.
 Quite seriously. Based on Planck's work, Einstein proposed that light also delivers
its energy in chunks; light would then consist of little particles, or quanta,
called photons, each with an energy of Planck's constant times its frequency.
Quite seriously. Based on Planck's work, Einstein proposed that light also delivers
its energy in chunks; light would then consist of little particles, or quanta,
called photons, each with an energy of Planck's constant times its frequency.
 In that case, the frequency of the light would make a difference in the
photoelectric effect.
In that case, the frequency of the light would make a difference in the
photoelectric effect.
 Exactly. Higher-frequency photons have more energy, so they should make the electrons
come flying out faster; thus, switching to light with the same intensity but a higher
frequency should increase the maximum kinetic energy of the emitted electrons. If you
leave the frequency the same but crank up the intensity, more electrons should
come out (because there are more photons to hit them), but they won't come out any
faster, because each individual photon still has the same energy.
Exactly. Higher-frequency photons have more energy, so they should make the electrons
come flying out faster; thus, switching to light with the same intensity but a higher
frequency should increase the maximum kinetic energy of the emitted electrons. If you
leave the frequency the same but crank up the intensity, more electrons should
come out (because there are more photons to hit them), but they won't come out any
faster, because each individual photon still has the same energy.
 And if the frequency is low enough, then none of the photons will have enough energy to
knock an electron out of an atom. So if you use really low-frequency light, you
shouldn't get any electrons, no matter how high the intensity is. Whereas if
you use a high frequency, you should still knock out some electrons even if the
intensity is very low.
And if the frequency is low enough, then none of the photons will have enough energy to
knock an electron out of an atom. So if you use really low-frequency light, you
shouldn't get any electrons, no matter how high the intensity is. Whereas if
you use a high frequency, you should still knock out some electrons even if the
intensity is very low.
 Quite right. Therefore, with a few simple measurements, the photoelectric effect would
seem to be able to tell us whether light is in fact made up of particles or waves.
Quite right. Therefore, with a few simple measurements, the photoelectric effect would
seem to be able to tell us whether light is in fact made up of particles or waves.
 So did someone do the experiment? Which way did it turn out?
So did someone do the experiment? Which way did it turn out?
 In 1913-1914, R.A. Millikan did a series of extremely careful experiments involving the
photoelectric effect. He found that all of his results agreed exactly with Einstein's
predictions about photons, not with the wave theory. Einstein actually won the Nobel
Prize for his work on the photoelectric effect, not for his more famous theory of
relativity.
In 1913-1914, R.A. Millikan did a series of extremely careful experiments involving the
photoelectric effect. He found that all of his results agreed exactly with Einstein's
predictions about photons, not with the wave theory. Einstein actually won the Nobel
Prize for his work on the photoelectric effect, not for his more famous theory of
relativity.
 Then light is made of particles! But wait...what about the two-slit experiment?
I don't see how light could make an interference pattern like that, unless it was made
of waves.
Then light is made of particles! But wait...what about the two-slit experiment?
I don't see how light could make an interference pattern like that, unless it was made
of waves.
 Yes, I'm afraid it's a bit more complicated than that. Some experimental results, like
this one, seem to prove beyond all possible doubt that light consists of particles;
others insist, just as irrefutably, that it's waves. We can only conclude that light
is somehow both a wave and a particle--or that it's something else we can't quite
visualize, which appears to us as one or the other depending on how we look at it.
Yes, I'm afraid it's a bit more complicated than that. Some experimental results, like
this one, seem to prove beyond all possible doubt that light consists of particles;
others insist, just as irrefutably, that it's waves. We can only conclude that light
is somehow both a wave and a particle--or that it's something else we can't quite
visualize, which appears to us as one or the other depending on how we look at it.





