Beyond the Hydrogen Atom
 I want to know more about elements other than hydrogen. You've said that each
element has its own set of spectral
lines, and claimed that these were explained by energy levels and quantum leaps and things, but we've
only really discussed the hydrogen atom.
I want to know more about elements other than hydrogen. You've said that each
element has its own set of spectral
lines, and claimed that these were explained by energy levels and quantum leaps and things, but we've
only really discussed the hydrogen atom.
 Ah, I admire your perspicacity. It's quite true, there are far more things in
heaven and earth than just hydrogen--over 114 different elements have now been
discovered or created in the laboratory. Let me show you an interactive
periodic table of elements, which goes up to number 36, krypton (Kr). The
table should already have opened in another window if your browser understands
javascript; if not, click here:
Ah, I admire your perspicacity. It's quite true, there are far more things in
heaven and earth than just hydrogen--over 114 different elements have now been
discovered or created in the laboratory. Let me show you an interactive
periodic table of elements, which goes up to number 36, krypton (Kr). The
table should already have opened in another window if your browser understands
javascript; if not, click here:
 to open it now.
to open it now.
 Hey, if you click on different elements, you can see their spectral lines, and
how many electrons they have. But what do s, p, and d mean?
Hey, if you click on different elements, you can see their spectral lines, and
how many electrons they have. But what do s, p, and d mean?
 Well...let's start from the top, with our old pal hydrogen; go ahead and
click on it in the table. It has just one proton, with one electron...I
won't say "orbiting" around it, because that's the discredited Rutherford model, but more or less
hovering around it in a fuzzy sort of way.
Well...let's start from the top, with our old pal hydrogen; go ahead and
click on it in the table. It has just one proton, with one electron...I
won't say "orbiting" around it, because that's the discredited Rutherford model, but more or less
hovering around it in a fuzzy sort of way.
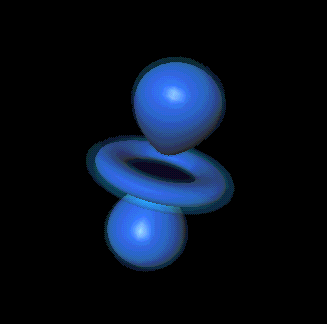
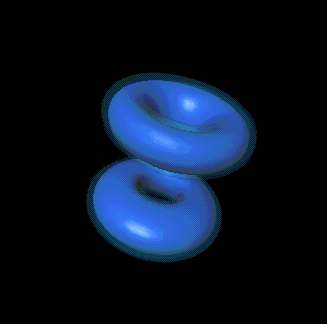
 You mean like in one of those probability cloud things in the Schrödinger model?
You mean like in one of those probability cloud things in the Schrödinger model?
|
|

|
 Precisely. Each cloud shape represents a different "state" of the electron.
We've talked about energy levels; the energy level of the electron is one of
the things that make one state different from another. Each level, or state,
has a specific energy for the electron and a specific set of probabilities for
its showing up in various places.
Precisely. Each cloud shape represents a different "state" of the electron.
We've talked about energy levels; the energy level of the electron is one of
the things that make one state different from another. Each level, or state,
has a specific energy for the electron and a specific set of probabilities for
its showing up in various places.

 And hydrogen's spectral lines are created when the electron jumps from one
level to another--I remember talking about all that stuff.
And hydrogen's spectral lines are created when the electron jumps from one
level to another--I remember talking about all that stuff.
 Okay, good. Now helium, the next element in line, has two protons, and, in
the neutral atom, two electrons...
Okay, good. Now helium, the next element in line, has two protons, and, in
the neutral atom, two electrons...
 I don't understand what you mean by "in the neutral atom." It doesn't
always have two electrons?
I don't understand what you mean by "in the neutral atom." It doesn't
always have two electrons?
 A given element always has the same number of protons, but the number of
electrons can vary. However, when an atom is
electrically neutral, as we've represented them here, it has just as many
electrons as protons. Now, click on helium in the periodic table in the other
window.
A given element always has the same number of protons, but the number of
electrons can vary. However, when an atom is
electrically neutral, as we've represented them here, it has just as many
electrons as protons. Now, click on helium in the periodic table in the other
window.
 I see the two electrons--but what I still don't understand is why helium's
spectrum is so different from hydrogen's. If it's created the same way, by
those electrons' jumping between energy levels, then the levels in helium must
not be the same as the ones in hydrogen.
I see the two electrons--but what I still don't understand is why helium's
spectrum is so different from hydrogen's. If it's created the same way, by
those electrons' jumping between energy levels, then the levels in helium must
not be the same as the ones in hydrogen.
 That's exactly right. The difference arises from the extra charged particles
that are now in the picture. First, each electron is being attracted to the
nucleus by twice as much positive charge as in hydrogen, and that changes the
set of energies that are "allowed." Meanwhile, the two negatively charged
electrons are repelling each other, and that also has some effect on the
allowed states.
That's exactly right. The difference arises from the extra charged particles
that are now in the picture. First, each electron is being attracted to the
nucleus by twice as much positive charge as in hydrogen, and that changes the
set of energies that are "allowed." Meanwhile, the two negatively charged
electrons are repelling each other, and that also has some effect on the
allowed states.
 Okay, I think I can see how this whole periodic table is built up. You just
add another proton and another electron, and you get a new element with
different spectral lines.
Okay, I think I can see how this whole periodic table is built up. You just
add another proton and another electron, and you get a new element with
different spectral lines.
 That's essentially correct. As you're about to see, though, the electrons
have to be added in a very particular way...
That's essentially correct. As you're about to see, though, the electrons
have to be added in a very particular way...






