Electron Clouds and Energy Levels
 Okay, so why is it that s only holds two electrons, but p has room for six?
Okay, so why is it that s only holds two electrons, but p has room for six?
 Well...remember that each electron is existing in one of
those strange probability clouds, which, as you've seen, can have widely
varying shapes and sizes. Another statement of the Pauli exclusion principle I mentioned is
this: no two electrons in an atom can be in the same type of cloud with the
same spin.
Well...remember that each electron is existing in one of
those strange probability clouds, which, as you've seen, can have widely
varying shapes and sizes. Another statement of the Pauli exclusion principle I mentioned is
this: no two electrons in an atom can be in the same type of cloud with the
same spin.
 So you're saying that p electrons have more cloud shapes available to them?
So you're saying that p electrons have more cloud shapes available to them?
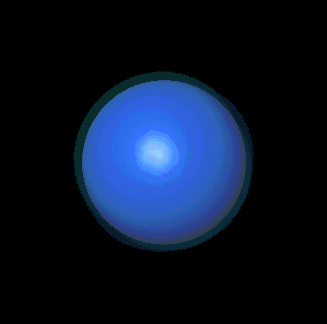
 Precisely. It happens that s clouds are always
spherical; the spheres just get bigger as the primary energy level
increases.
Precisely. It happens that s clouds are always
spherical; the spheres just get bigger as the primary energy level
increases.
|
|
However, p and d states are more interesting: there can be several
different-shaped clouds at the same energy.
For example, here are
two p states from the second primary level:
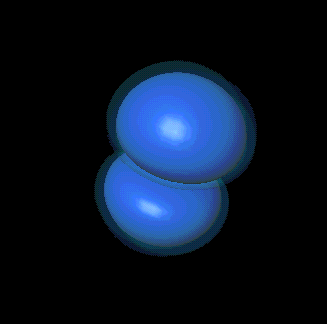
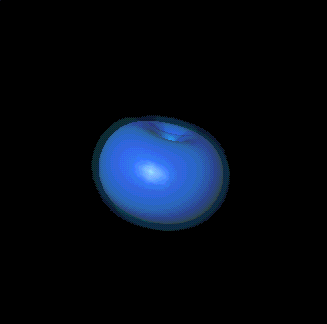
It turns out that there are three kinds of p clouds in each
primary level.
 ...and each one can hold a spin up and a spin down, so that's why six
electrons fit into the p column!
...and each one can hold a spin up and a spin down, so that's why six
electrons fit into the p column!
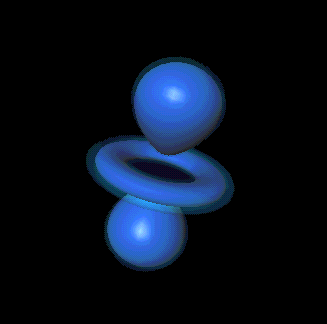
 Very good. You can think of each electron's "quantum
state," its full, unique description, as being the sum of a particular
probability cloud plus a spin:
Very good. You can think of each electron's "quantum
state," its full, unique description, as being the sum of a particular
probability cloud plus a spin:
|
|

|
|
|
|
 Futhermore, there are five different shapes for d (hence room for ten
electrons), and seven in the next sublevel, f...I hope you're noticing a
pattern here. (If you want to know more about where these numbers come from,
go ask Dr. Mahan about quantum numbers.)
Futhermore, there are five different shapes for d (hence room for ten
electrons), and seven in the next sublevel, f...I hope you're noticing a
pattern here. (If you want to know more about where these numbers come from,
go ask Dr. Mahan about quantum numbers.)






