Quantum Numbers
 Each electron has a set of four numbers, called quantum numbers, that
specify it completely; no two electrons in the same atom can have the same
four. That's a more precise statement of the Pauli exclusion principle Bob was
discussing. (He also mentioned still another
way of expressing this important idea.)
Each electron has a set of four numbers, called quantum numbers, that
specify it completely; no two electrons in the same atom can have the same
four. That's a more precise statement of the Pauli exclusion principle Bob was
discussing. (He also mentioned still another
way of expressing this important idea.)
 Is there a special reason why there are four, and not three or six or
fifty-nine?
Is there a special reason why there are four, and not three or six or
fifty-nine?
 Good question. There are certainly reasons, but I won't be able to explain
them to you here, any more than Bob could explain where his rules were coming
from. What I can offer you is a mathematical expression of those rules, which
I hope will make them easier to work with and perhaps provide some insight
into the underlying patterns.
Good question. There are certainly reasons, but I won't be able to explain
them to you here, any more than Bob could explain where his rules were coming
from. What I can offer you is a mathematical expression of those rules, which
I hope will make them easier to work with and perhaps provide some insight
into the underlying patterns.
 Okay, I can live with that. Tell me about the four numbers.
Okay, I can live with that. Tell me about the four numbers.
 First, the "primary quantum number," which is given the symbol n, corresponds to those colored rows you saw in the
chart. The lowest row, the pink one, has electrons with n=1; the
yellow row is n=2, and they go up from there.
First, the "primary quantum number," which is given the symbol n, corresponds to those colored rows you saw in the
chart. The lowest row, the pink one, has electrons with n=1; the
yellow row is n=2, and they go up from there.
 All right, so n tells you which of the "main" energy levels you're in.
I suppose there's another quantum number that goes with the sublevels--s, p, d, and all that.
All right, so n tells you which of the "main" energy levels you're in.
I suppose there's another quantum number that goes with the sublevels--s, p, d, and all that.
 Very good. The second quantum number is known as l. A value of
l=0 corresponds to s, l=1 is p, l=2 is d, and so forth.
Very good. The second quantum number is known as l. A value of
l=0 corresponds to s, l=1 is p, l=2 is d, and so forth.
 This all seems very abstract to me. What does l really mean?
Can you give me some concrete way to think about it?
This all seems very abstract to me. What does l really mean?
Can you give me some concrete way to think about it?
 I have two answers for that. First, l, unlike n, does have an
association with angular momentum. If you'd like to know more about this,
click on the "advanced" button at right.
I have two answers for that. First, l, unlike n, does have an
association with angular momentum. If you'd like to know more about this,
click on the "advanced" button at right.
|

|
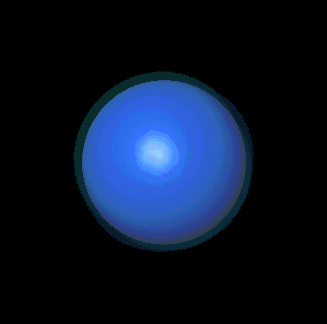
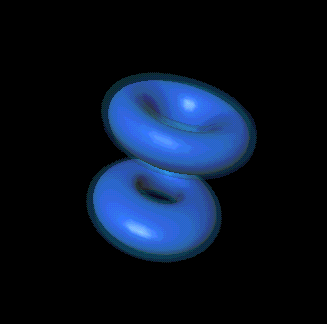
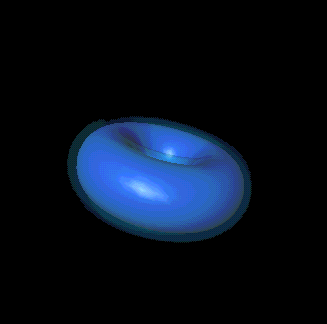
If "angular momentum" means nothing to you, don't despair. You can also
picture its significance this way: l, along with n and the third
quantum number, m, is responsible for determining the shape of an
electron's probability cloud. Here are a few examples:
|
|
|

|
|
|
|
|
|






