Evidence for a Nucleus
 One of Thomson's most radical (and, as it turned out, best) ideas was to
suggest that the electrons he'd discovered were part of the chemists' atoms.
This contradicted Dalton's idea that atoms were "uncuttable," with no
smaller parts. It did seem to make sense, though: Thomson knew that
electric currents can be started by chemical reactions (as in a battery) and
by light shining on metal, in the photoelectric effect, so
electrons must have some connection with the world of atoms.
One of Thomson's most radical (and, as it turned out, best) ideas was to
suggest that the electrons he'd discovered were part of the chemists' atoms.
This contradicted Dalton's idea that atoms were "uncuttable," with no
smaller parts. It did seem to make sense, though: Thomson knew that
electric currents can be started by chemical reactions (as in a battery) and
by light shining on metal, in the photoelectric effect, so
electrons must have some connection with the world of atoms.
But if atoms have electrons in them, why are they electrically neutral?
 They have to have a positively charged part, to cancel out the negative
electrons.
They have to have a positively charged part, to cancel out the negative
electrons.
 Exactly. Thomson's original picture of the atom was
called the "plum pudding model," though, in the spirit of the modern
age, I prefer to think of it as the "chocolate
chip cookie model." The electrons were the "chips," embedded in a
positively charged hunk of "cookie dough." Exactly. Thomson's original picture of the atom was
called the "plum pudding model," though, in the spirit of the modern
age, I prefer to think of it as the "chocolate
chip cookie model." The electrons were the "chips," embedded in a
positively charged hunk of "cookie dough."
|

|
| In 1911, Ernest Rutherford announced experimental evidence showing that the cookie model was wrong. |

|
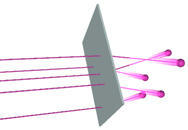
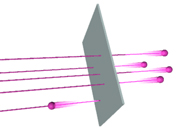
| Rutherford's experiments consisted of shooting alpha particles at thin sheets of metal. He then measured the angles at which they came sailing out. |
 So what happened?
So what happened?
 Based on Thomson's model, Rutherford assumed the flying, positively charged
alpha particles would be pushed a little to the side by the positive "cookie
dough" in the metal atoms, and continue flying along at a slightly different
angle. He was shocked by what he actually saw. Most of the alpha particles
went right through the metal without changing course at all, but a few turned
a full 180 degrees and went shooting back the way they'd come.
Based on Thomson's model, Rutherford assumed the flying, positively charged
alpha particles would be pushed a little to the side by the positive "cookie
dough" in the metal atoms, and continue flying along at a slightly different
angle. He was shocked by what he actually saw. Most of the alpha particles
went right through the metal without changing course at all, but a few turned
a full 180 degrees and went shooting back the way they'd come.
 |
 |
Rutherford compared the experience to shooting an artillery shell at a
piece of tissue paper--and seeing the shell bounce back.

 And that meant the cookie picture was wrong?
And that meant the cookie picture was wrong?
 If the positive charge were spread throughout the whole atom, as
in the cookie model, Rutherford calculated that there would be no
possibility of the particles bouncing back that way. The only way his
results made sense was if he assumed that all the positive charge, and
almost all the atom's mass, was concentrated in a tiny lump at the
center--what we now call the nucleus. He imagined the electrons orbiting
around the nucleus like planets around the sun, with a (relatively) huge
empty space between them.
If the positive charge were spread throughout the whole atom, as
in the cookie model, Rutherford calculated that there would be no
possibility of the particles bouncing back that way. The only way his
results made sense was if he assumed that all the positive charge, and
almost all the atom's mass, was concentrated in a tiny lump at the
center--what we now call the nucleus. He imagined the electrons orbiting
around the nucleus like planets around the sun, with a (relatively) huge
empty space between them.
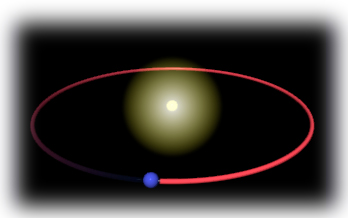
Rutherford's model had a few problems, which helped inspire the development
of quantum mechanics--I've told that story more fully elsewhere. The current picture of
the atom has the electrons not orbiting but in "clouds" at different energy
levels--see the Schrödinger
Model or the Elements as Atoms
section for more details.






